Our New FDA-Approved Treatment: Learn More
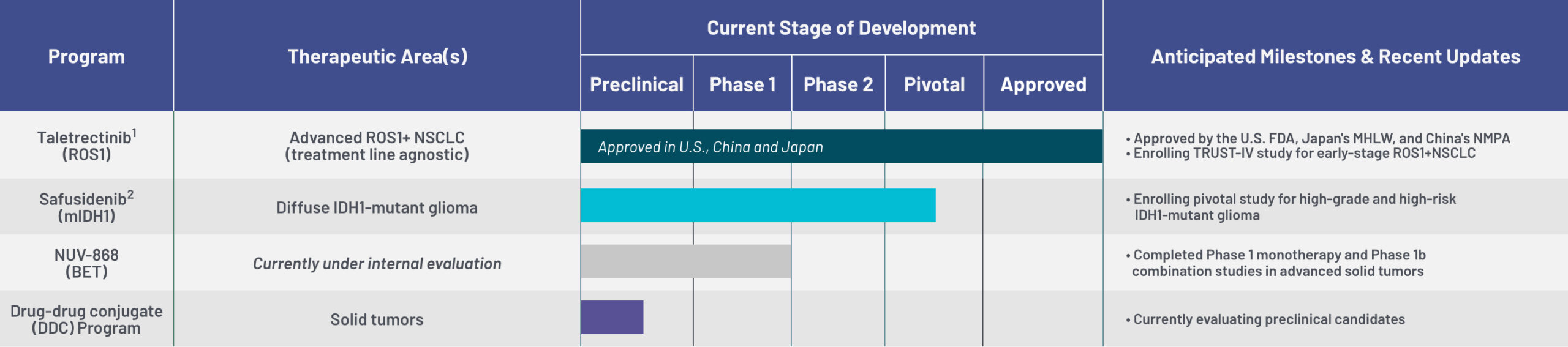
Nuvation Bio is focused on tackling some of the toughest challenges in cancer treatment. Our clinical trial programs include taletrectinib (ROS1 inhibitor), safusidenib (mIDH1 inhibitor), NUV-868 (BET inhibitor), and our Drug-Drug Conjugate (DDC) program.
Program
Therapeutic Area(s)
Current Stage of Development
Preclinical
Phase 1
Phase 2
Pivotal
Approved
Anticipated Milestones & Recent Updates
Taletrectinib1
(ROS1)
Advanced ROS1+ NSCLC
(treatment line agnostic)
• Approved by the U.S. FDA, Japan's MHLW, and China's NMPA
• Enrolling TRUST-IV study for early-stage ROS1+NSCLC
Safusidenib2
(mIDH1)
Diffuse IDH1-mutant glioma
• Enrolling pivotal study for high-grade and high-risk
IDH1-mutant glioma
NUV-868
(BET)
Currently under internal evaluation
• Completed Phase 1 monotherapy and Phase 1b
combination studies in advanced solid tumors
Drug-drug conjugate
(DDC) Program
Solid tumors
• Currently evaluating preclinical candidates
NUV-868
(BET)
Advanced solid
tumors3
NUV-868 +
olaparib
Phase 1b dose escalation study ongoing
BET: Bromodomain and Extra-Terminal motif; ESMO: European Society of Medical Oncology Congress; MHLW: Ministry of Health, Labour and Welfare; mIDH1: mutant isocitrate dehydrogenase 1; NSCLC: Non-small cell lung cancer; ROS1+: c-ros oncogene 1-positive. 1. Worldwide development and commercial rights in-licensed from Daiichi Sankyo; rights to IBTROZI have been out-licensed in China (Innovent Biologics), Japan (Nippon Kayaku), Europe and additional regions (Eisai). 2. Worldwide development and commercial rights in-licensed from Daiichi Sankyo, excluding Japan where Daiichi Sankyo retains development and commercial rights. Pivotal study includes patients with grade 4 astrocytoma and patients with grade 2 or 3 astrocytoma with certain high-risk features.
Each year, more than one million people globally are diagnosed with NSCLC, the most common form of lung cancer. It is estimated that approximately 2% of people with NSCLC have ROS1+ disease. About 35% of people newly diagnosed with metastatic ROS1+ NSCLC have tumors that have spread to their brain. The brain is also the most common site of disease progression, with about 50% of previously treated patients developing central nervous system metastases. Despite recent progress for patients with ROS1+ NSCLC, there remains a need for treatment options that address some of the outstanding challenges of treating the disease.
Safusidenib is being evaluated in a pivotal, Phase 3 study for the treatment of patients with high-grade and high-risk IDH1-mutant glioma. Safusidenib has shown high blood-brain barrier penetration in both pre-clinical and clinical studies and demonstrated anti-tumor activity and tolerability in Phase 1 and 2 clinical studies. Gliomas are the most common type of adult brain cancer worldwide. In the U.S., nearly 2,400 people are diagnosed with IDH1-mutant gliomas each year. Most patients are diagnosed in their 30s and 40s. While patients with IDH1 mutations generally have longer survival times than those with wild-type IDH1, gliomas are not currently curable and prognosis worsens for those with high grade tumors.
NUV-868 is almost 1,500 times more selective for BD2 than BD1 and is designed to alleviate the therapeutic limiting toxicities observed by other non-BD2 selective BET inhibitors.
We have completed a Phase 1 monotherapy study and a Phase 1b study of NUV-868 in combination with olaparib or enzalutamide. We are evaluating next steps for the NUV-868 program, including further development in combination with approved products for indications in which BD2-selective BET inhibitors may improve outcomes for patients.
The platform is designed to selectively deliver potent anti-cancer therapeutics to cancer cells to exert greater toxicity against these target tumor cells than against healthy non-target tissues.
Utilizing this technology, we are able to design potent oncology-focused chimeric small molecules which combine tumor-targeting specificity with anti-cancer activity of known oncology agents. We believe our DDC technology will be broadly applicable and can be replicated across many existing therapies to transform the standard-of-care in multiple oncology indications.
At Nuvation Bio, we are committed to providing a safe and secure experience for job seekers. All official communication regarding employment opportunities will come exclusively via email from the official nuvationbio.com domain. We do not conduct interviews through texting applications or instant messaging platforms. If you receive an employment offer or other correspondence from an individual at Nuvation Bio you have not met, or from a non-Nuvation Bio email domain, it may be fraudulent.
To ensure security online and protect against potential fraudulent job offers, please familiarize yourself with the resources available on the Federal Trade Commission’s Job Scams page. Exercise caution and refrain from interacting with unverified domains that are not associated with our official company domain: NuvationBio.com. For reliable job information about Nuvation Bio open positions, visit our Careers page, and reach out to hr@nuvationbio.com if you have any concerns.
Nuvation Bio is a global oncology company focused on tackling some of the toughest challenges in cancer treatment.
info@nuvationbio.com
media@nuvationbio.com
IR@nuvationbio.com
© 2026 NUVATION BIO INC. ALL RIGHTS RESERVED. TERMS AND CONDITIONS | PRIVACY